

THESE SYMPTOMS MAY BE AN INDICATION THAT SOMETHING IS WRONG WITH YOUR PANCREAS1
The pancreas is an organ located behind the stomach, responsible for producing enzymes for digestion.3
In addition, the pancreas produces hormones to regulate blood sugar levels in the body.3
Although a healthy pancreas is only roughly the size of a hand,3 it produces enough enzymes to break down food so that it can be absorbed by the gut into the bloodstream and used by the body.3 It therefore plays a major role in maintaining a healthy nutritional status.1,3,4
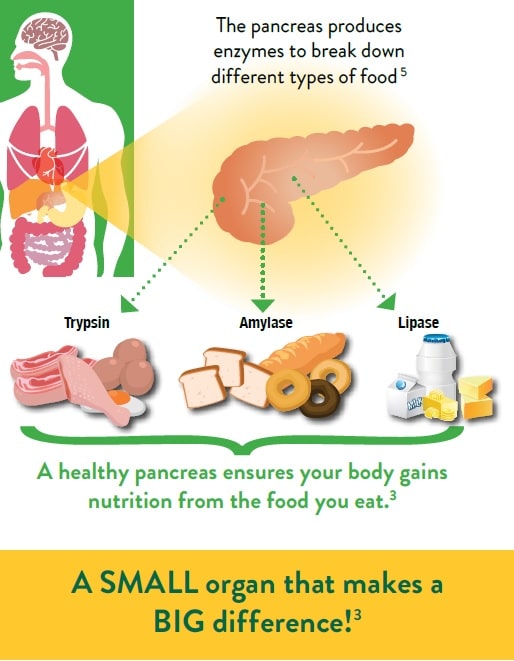
A disorder called Pancreatic Exocrine Insufficiency (PEI) can occur when your pancreas does not produce enough enzymes to break down the food you eat.6
Without these enzymes, nutrients from food are not absorbed by the body.6
Although impaired pancreatic function could be hereditary, there are other conditions that could affect the pancreas, preventing it from producing the enzymes needed to function properly.2
These conditions include inflammation of the pancreas, cystic fibrosis or if your pancreas has been removed or affected by surgery.2
Loss of pancreatic function usually results in poor fat, carbohydrate and protein absorption. Vitamins A, D, E and K are fat soluble, these are also poorly absorbed.2
Yes, PEI can be treated!
The main goal of treating PEI is to replace the enzymes that your pancreas is not producing, to ensure correct digestion of the food you eat, and absorption of the nutrients.6
This treatment is known as pancreatic enzyme replacement therapy (PERT).6
CREON® capsules should be taken at the start of meals and snacks
Capsules should be swallowed whole, not chewed
Alternatively open the capsule, pour the pellets onto a spoon and swallow without chewing
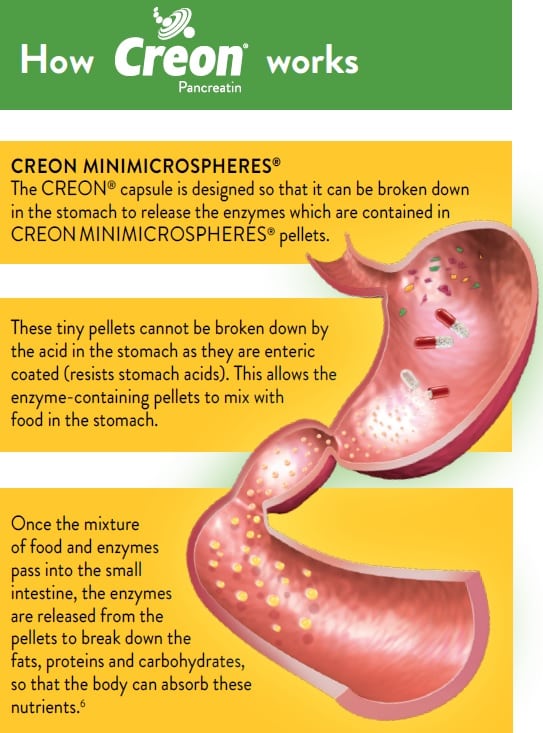
The correct dose of CREON® depends on your condition and symptoms.6
Your doctor or pharmacist will advise on the appropriate dose for you.
Creon® 10000. Each capsule contains Pancreatin 150 mg. Reg. No.: 33/11.1/0340; Mauritius POM Reg. No.:
R7435/02/16; Namibia: Reg. No.: 04/11.1/1015. Creon® 25000. Each capsule contains Pancreatin 300 mg.
Reg. No.: 28/11.1/0645; Namibia: Reg. No.: 04/11.1/1016.
For full prescribing information refer to the Professional Information approved by the Medicines Regulatory Authority.
Abbott Laboratories S.A. (Pty) Ltd. 1940/014043/07. Abbott Place, 219 Golf Club Terrace, Constantia Kloof, 1709.
Publication date: July 2022. Promotional review number: SAF2235118.
Disclaimer
This e-brochure has been auto-translated for your convenience. While machine translations are helpful, they may contain errors. Medinformer is actively working to have all translations reviewed by professional, mother-tongue language speakers, though this process will take time. For the most accurate information, please refer to the original English version. Medinformer and its partners cannot be held responsible for any inaccuracies that may result from the translation.
Thank you for your understanding.